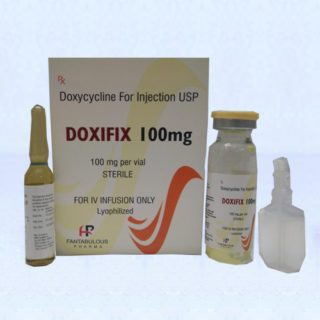
Doxycycline injection manufacturers
Product Description
Doxycycline injection Manufacturers In India | Third Party Manufacturers For Doxycycline Manufacturers | Doxycycline injectable Manufacturers | Doxycycline injections Manufacturers
Doxycycline injection manufacturers :-
Doxycycline injection Manufacturers USP is a sterile, lyophilized powder prepared from a solution of doxycycline hyclate, ascorbic acid and mannitol in Water for Injection. Doxycycline hyclate is a broad spectrum antibiotic derived from oxytetracycline. It is meant for INTRAVENOUS use only after reconstitution
Tetracyclines are readily absorbed and are bound to plasma proteins in varying degree. They are concentrated by the liver in the bile, and excreted in the urine and feces at high concentrations and in a biologically active form. Doxycycline is virtually completely absorbed after oral administration.
Use of Doxycycline injection
Following a 200 mg dose, normal adult volunteers averaged peak serum levels of 2.6 mcg/mL of doxycycline at 2 hours, decreasing to 1.45 mcg/mL at 24 hours. Excretion of doxycycline by the kidney is about 40 %/72 hours in individuals with normal function (creatinine clearance about 75 mL/min). This percentage excretion may fall as low as 1 to 5 %/72 hours in individuals with severe renal insufficiency (creatinine clearance below 10 mL/min). Studies have shown no significant difference in serum half-life of doxycycline (range 18 to 22 hours) in individuals with normal and severely impaired renal function.
A report of Intermediate (I) indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug product is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone that prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of Resistant (R) indicates that the antimicrobial drug is not likely to inhibit growth of the microorganism if the antimicrobial drug reaches the concentrations usually achievable at the infection site; other therapy should be selected.